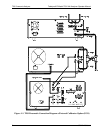
Teledyne API Model T201 NH
3
Analyzer Operator Manual T201 Ammonia Analyzer
07271B DCN6646 11
The ammonia converter efficiency factor (NH
3
_CE_FACTOR1) is
discussed in more detail in Section 2.3 of this manual. The conversion
efficiency of the M501 NH
3
should be checked prior to starting long term
tests. Both efficiency factors can be accessed through the analyzer VARS
MENU.
The actual formula for computation of the gas concentrations is more
complicated than the above equations, as it includes sample, reaction cell
pressure changes and averaging the PMT signal. Then the zero offset and
slopes are applied to the separate channels (TN
x
, NO
x
, NO) to determine
the concentrations. Concentration compensation occurs while the variable
TP_FACTOR under the VARS MENU is switched ON. Otherwise the
displayed concentration is uncompensated. It is recommended that
the variable TP_FACTOR remain on at all times.
1.1.1 Minimizing PMT Drift
In order to account for PMT drift in the analyzer, the AutoZero valve
switches once a minute allowing the analyzer to read zero background.
The AutoZero valve directs the sample gas stream to completely bypass
the reaction cell, while simultaneously filling the reaction cell with Ozone
for dark noise measurement. This is then subtracted as a measurement
offset from the raw PMT concentration signal. This process improves zero
baseline stability by minimizing the effect of PMT sensor drift.
1.1.2 Purging the Reaction Cell
As with many chemical reactions the conversion of ammonia in the
presence of other oxides of nitrogen is complicated. It is important to note
that the valve DWELL time for an AZERO measurement has a default
setting of 8 seconds. Shortening this value may not allow enough time to
properly purge the reaction cell of excess nitric oxide from the previous
measurements.
In the molybdenum converter operating at 315
o
C the following significant
reactions are taking place:
Mo + NO
2
MoO
3
+ NO ~100% Efficiency
The M501 NH
3
ammonia converter operates at 825
o
C. At this high
temperature, several reactions occur:
NO NO Loss = ~ 3%
NO
2
NO Efficiency = ~97%
NH
3
NO Efficiency = ~97%
NH
3
NO
2
Efficiency = ~5%